ZOOHCC - 501: Molecular Biology (Theory)
Unit 3: Transcription and Regulatory RNAs
Unit 3: Transcription and Regulatory RNAs
Transcription in Eukaryotes
Prokaryotes and eukaryotes perform basically the same transcription
process, but there are some important differences. The most important
difference between prokaryotes and eukaryotes is the membrane-bound nucleus
and organelles of the latter. Eukaryotic cells, which have genes
encapsulated in the nucleus, must be able to transport mRNA to the cytoplasm
and must protect the mRNA from degradation before it is translated.
Eukaryotes also use three different polymerases, each of which transcribes a
different subset of genes.
Initiation
The eukaryotic promoters that we are most interested in are similar to
prokaryotic promoters in that they contain a TATA box (Figure 1). However,
transcription initiation in eukaryotes is much more complex than in
prokaryotes. Unlike prokaryotic RNA polymerase, which can itself bind to a
DNA template, eukaryotes require several other proteins, called
transcription factors, to first bind to the promoter region and then recruit
the appropriate polymerase. help you to
In addition, there are three different RNA polymerases in eukaryotes, each
composed of ten or more subunits. Each eukaryotic RNA polymerase also
requires a specific set of transcription factors to reach the DNA
template.
RNA polymerase I
RNA polymerase I resides in the nucleolus, a specialized nuclear
substructure where ribosomal RNA (rRNA) is transcribed, processed, and
assembled into ribosomes. rRNA molecules are considered structural RNAs
because they perform cellular functions but are not translated into
proteins. rRNA is a component of the ribosome and is essential for the
translation process. RNA polymerase I synthesizes most rRNA.
RNA polymerase II
RNA polymerase II resides in the cell nucleus and synthesizes all nuclear
pre-mRNAs that code for proteins. Eukaryotic pre-mRNAs undergo extensive
post-transcriptional and pre-translational processing. For clarity, the term
'mRNA' is only used to denote the mature, processed molecule that is ready
for translation. RNA polymerase II is involved in the transcription of most
eukaryotic genes.
RNA polymerase III
RNA polymerase III is also located in the cell nucleus. This polymerase
transcribes a variety of structural RNAs, including transfer pre-RNAs
(pre-tRNAs) and small core pre-RNAs. tRNAs play an important role in
translation. They act as adapter molecules between the mRNA template and the
growing polypeptide chain. Small nuclear RNAs have a variety of functions,
including 'splicing' pre-mRNAs and regulating transcription factors.
Each type of RNA polymerase recognizes different promoter sequences and
requires different transcription factors.
Elongation
After formation of the pre-initiation complex, the polymerase is released
from other transcription factors, elongation proceeds in a manner similar to
prokaryotes, and RNA polymerase synthesizes mRNA in the 5' to 3' direction.
As mentioned earlier, RNA polymerase II transcribes the majority of
eukaryotic genes, so this section will focus on how this polymerase
accomplishes elongation and termination.
Although the enzymatic elongation process is essentially the same in
eukaryotes and prokaryotes, the DNA template is more complex. When
eukaryotic cells are not dividing, their genes exist as a diffuse mass of
DNA and protein called chromatin. DNA clusters around charged histone
proteins at repeated intervals. Collectively called nucleosomes, these
DNA-histone complexes are regularly spaced and consist of 146 DNA
nucleotides wrapped around eight histones like threads around a spool.
For RNA synthesis to take place, histones must be eliminated each time the
transcription machinery encounters a nucleosome. This is achieved by a
special protein complex called FACT. It stands for “promoting chromatin
transcription.” This complex pulls the histone away from the DNA template as
the polymerase moves along it. Once the pre-mRNA is synthesized, the FACT
complex replaces histones to reassemble the nucleosome.
Termination
Termination of transcription depends on the polymerase. In eukaryotes,
unlike in prokaryotes, extension by RNA polymerase II occurs 1,000–2,000
nucleotides after the end of the transcribed gene. This pre-mRNA tail is
removed during mRNA processing. RNA polymerases I and III require
termination signals. Genes transcribed by RNA polymerase I contain a
specific 18-nucleotide sequence that is recognized by a termination protein.
The termination process of RNA polymerase III involves an mRNA hairpin that
triggers the release of the mRNA
Eukaryotic RNA Polymerases and General Transcription Factors
Although the basic mechanisms of transcription are the same in all cells,
they are considerably more complex in eukaryotic cells than in bacteria.
This is reflected in her two distinct differences between prokaryotic and
eukaryotic systems. First, all genes in bacteria are transcribed by a single
RNA polymerase, whereas eukaryotic cells contain several different RNA
polymerases that transcribe different classes of genes. Second, eukaryotic
RNA polymerases must specifically initiate transcription by interacting with
various additional proteins rather than directly binding to promoter
sequences. This increased complexity of eukaryotic transcription likely
facilitates the sophisticated regulation of gene expression necessary to
direct the activities of many different cell types in multicellular
organisms..
Protein involvement in prokaryotic transcription
Eukaryotic cells contain three different nuclear RNA polymerases that
transcribe different classes of genes (Table 6.1). Protein-coding genes are
transcribed by RNA polymerase II to produce mRNA. Ribosomal RNA (rRNA) and
transfer RNA (tRNA) are transcribed by RNA polymerases I and III. RNA
polymerase I specializes in transcription of the three largest types of
rRNA, named 28S, 18S, and 5.8S, depending on their sedimentation velocities
during high-speed centrifugation. RNA polymerase III transcribes genes to
tRNA and minimal ribosomal RNA (5S rRNA). Some of the small RNAs (snRNAs and
scRNAs) involved in splicing and protein transport are also transcribed by
RNA polymerase III, while others are polymerase II transcripts. In addition,
another RNA polymerase (similar to that of bacteria) is found in
chloroplasts and mitochondria and specifically transcribes the DNA of these
organelles.
All three nuclear RNA polymerases are complex enzymes, each composed of
8–14 different subunits. They recognize different promoters and transcribe
different classes of genes, but share some common features. The two largest
subunits of all three eukaryotic RNA polymerases are related to the single
E. coli RNA polymerase β and β' subunits. Furthermore, all three different
enzymes share five subunits of eukaryotic RNA polymerases. Consistent with
these structural similarities, the various eukaryotic polymerases share
several functional properties, such as the need to interact with other
proteins to properly initiate transcription.
General Transcription Factors and Initiation of Transcription by RNA
Polymerase II
RNA polymerase II is the focus of most transcriptional studies in
eukaryotes because it is responsible for synthesizing mRNA from
protein-coding genes. Early attempts to study this enzyme showed that its
activity differs from that of prokaryotic RNA polymerases. Accurate
transcription of bacterial genes can be achieved in vitro by simply adding
purified RNA polymerase to promoter-containing DNA, but is not possible in
eukaryotic systems. The basis for this difference was elucidated in 1979
when Robert Roeder and his colleagues discovered that RNA polymerase II
could initiate transcription only when additional proteins were added to the
reaction. Thus, transcription in eukaryotic systems appeared to require
various initiation factors that are not associated with polymerases (in
contrast to bacterial σ factors).
Biochemical fractionation of nuclear extracts has identified specific
proteins (called transcription factors) required for RNA polymerase II to
initiate transcription. Indeed, the identification and characterization of
these factors represent a major part of ongoing efforts to understand
transcription in eukaryotic cells. Two general types of transcription
factors have been defined. General transcription factors form part of the
basic transcription machinery as they are involved in transcription from all
polymerase II promoters. Additional transcription factors (described later
in this chapter) are involved in the regulation of gene expression as they
bind to DNA sequences that control the expression of individual genes.
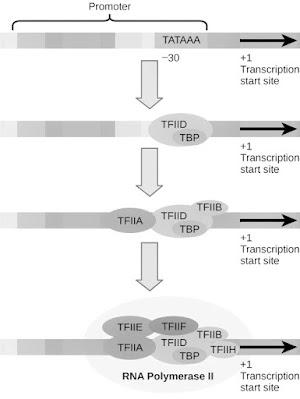
Initiation of transcription by RNA polymerase II in the reconstituted in
vitro system requires five general transcription factors (Figure 6.12). The
promoters of many genes transcribed by polymerase II contain a TATAA-like
sequence 25-30 nucleotides upstream of the transcription start site. This
sequence (termed the TATA box) resembles the -10 sequence element of
bacterial promoters, and mutations in the TATAA sequence demonstrated their
role in transcription initiation. The first step in transcription complex
formation is the binding of a common transcription factor called TFIID to
his TATA box (TF stands for transcription factor and II for polymerase II).
TFIID itself is composed of multiple subunits, including the TATA-binding
protein (TBP), which specifically binds to the TATAA consensus sequence, and
10–12 other polypeptides called TBP-associated factors (TAFs). TBP then
binds to a second common transcription factor (TFIIB) to form a TBP-TFIIB
complex at the promoter (Figure 6.13). TFIIB then acts as a bridge to RNA
polymerase, which, along with her third factor, TFIIF, binds to her
TBP-TFIIB complex.
After recruitment of RNA polymerase II to the promoter, transcription
initiation requires the binding of two additional factors (TFIIE and TFIIH).
TFIIH is a multi-subunit factor that appears to play at least two important
roles. First, her two subunits of TFIIH are helicases and can unwrap DNA
around the initiation site. (These subunits of TFIIH are also required for
nucleotide excision repair, as discussed in Chapter 5.) Another subunit of
TFIIH is the repeat located in the C-terminal domain of the largest subunit
of RNA polymerase II. A protein kinase that phosphorylates sequences.
Phosphorylation of these sequences is thought to disassociate the polymerase
from the initiation complex and allow it to proceed down the template,
elongating the growing RNA strand.
In addition to the TATA box, the promoters of many genes transcribed by RNA
polymerase II contain a second critical sequence element (initiator or Inr
sequence) spanning the transcription start site. In addition, some RNA
polymerase II promoters contain only Inr elements without TATA boxes.
Initiation at these promoters still requires TFIID (and TBP), but TBP does
not appear to recognize these promoters by binding directly to her TATA
sequences. Instead, other TFIID subunits (TAFs) appear to bind to her Inr
sequences. This binding recruits TBP to the promoter and assembles TFIIB,
polymerase II, and additional transcription factors as previously described.
Thus, TBP plays a central role in the initiation of polymerase II
transcription, even on promoters lacking a TATA box.
Despite the development of in vitro systems and the characterization of
several general transcription factors, much remains unknown about the
mechanisms of polymerase II transcription in eukaryotic cells. The
sequential recruitment of transcription factors described here represents a
minimal system required for transcription in vitro. Additional factors may
be required within the cell. Furthermore, RNA polymerase II appears to be
able to associate with several transcription factors in vivo before the
transcription complex is assembled on DNA. In particular, preformed
complexes of RNA polymerase II with TFIIB, TFIIE, TFIIF, TFIIH and other
transcriptional regulatory proteins have been detected in both yeast and
mammalian cells. These large complexes, called polymerase II holoenzymes,
are recruited to promoters by direct interaction with TFIID (Figure 6.14).
Therefore, the relative contribution of the stepwise assembly of individual
factors to the recruitment of the RNA polymerase II holoenzyme to promoters
in cells remains to be determined.
Transcription by RNA Polymerases I and III
As previously mentioned, various RNA polymerases are involved in the
transcription of genes encoding eukaryotic ribosomes and transfer RNAs.
However, all three RNA polymerases require additional transcription factors
to associate with appropriate promoter sequences. Furthermore, although the
three different polymerases recognize different types of promoters in
eukaryotic cells, a common transcription factor, the TATA-binding protein
(TBP), appears to be required for transcription initiation by all three
enzymes. .
RNA polymerase I is dedicated to transcription of ribosomal RNA genes
present in tandem repeats. Transcription of these genes produces a large 45S
pre-rRNA, which is processed to produce 28S, 18S, and 5.8S rRNA (Figure
6.15). The promoter of the ribosomal RNA gene extends approximately 150 base
pairs immediately upstream of the transcription start site. These promoter
sequences are recognized by two transcription factors, UBF (upstream binding
factor) and SL1 (selectivity factor 1), which cooperatively bind to the
promoter and recruit polymerase I to form the initiation complex. (Figure
6.16). The SL1 transcription factor is composed of four protein subunits,
one of which surprisingly is TBP. The role of TBP was directly demonstrated
by the finding that yeast harboring mutations in TBP are defective not only
in transcription by polymerase II, but also in transcription by polymerases
I and III. Thus, TBP is a common transcription factor required for all three
classes of eukaryotic RNA polymerases. Because the promoters of ribosomal
RNA genes do not contain TATA boxes, TBP does not bind to specific promoter
sequences. Instead, the binding of TBP to ribosomal RNA genes is mediated by
the binding of other proteins of the SL1 complex to their promoters. This is
a similar situation to the binding of TBP with the Inr sequence of the
polymerase II gene, where the TATA box is missed.
tRNA, 5S rRNA, and several small RNA genes involved in splicing and protein
transport are transcribed by polymerase III. These genes are characterized
by promoters located internal to the transcribed sequence rather than
upstream. The most thoroughly studied gene transcribed by polymerase III is
his 5S rRNA gene in Xenopus laevis. TFIIIA, the primary purified
transcription factor, initiates assembly of the transcription complex by
binding to specific DNA sequences in the 5S rRNA promoter. This binding is
followed by the sequential binding of TFIIIC, TFIIIB, and polymerase. The
tRNA gene promoter differs from the 5S rRNA promoter in that it does not
contain the DNA sequence recognized by TFIIIA. Instead, TFIIIC binds
directly to the promoter of the tRNA gene and recruits TFIIIB and polymerase
to form a transcription complex. TFIIIB is composed of several subunits, of
which (again) the TATA-binding protein is TBP. Although her three RNA
polymerases in eukaryotic cells recognize different promoters, TBP appears
to be the common element linking promoter recognition to polymerase
recruitment to transcription complexes.